There are many occasions where it might be helpful to know how much time it will take to heat or cool your system to a certain temperature. Or, you may want to calculate how much power is required to heat or cool a given volume of fluid in a certain amount of time.
Thankfully, there is a fairly simple equation you can use as long as you know the mass of the bath fluid, it’s specific heat capacity, the temperature differential, and either power or time.
That said, using this equation isn’t entirely reliable, as there are various factors that could throw off the calculation. In this post, we take a look at the equation for calculating heating or cooling time and the reasons you should look for a system with slightly more power than you think you need.
Calculating Heating or Cooling Time
You can use the same basic equation when calculating heating or cooling time, although there is a little more work involved for calculating cooling time. When heating, the power applied is constant, but when cooling, the power (or the cooling capacity) is variable depending on the temperature.
Calculating Heating Time
To find out how much time it will take to heat a bath to a certain temperature, you can use the following equation:
t = mcΔT / P
Where:
- t is heating or cooling time in seconds
- m is the mass of the fluid in kilograms
- c is the specific heat capacity of the fluid in joules per kilogram and per Kelvin
- ΔT is the temperature differential in degrees Celcius or Fahrenheit
- P is the power at which energy is supplied in watts or joules per second
Similarly, to calculate the power needed to heat or cool a bath to a certain temperature in a given time, you can use this equation:
P = mcΔT / t
While these equations are fairly straightforward to follow, there can be some confusion when it comes to which units to use. Instead, you could use an online calculator to help.
This calculator is nice and simple and lets you calculate time, power, or energy consumed, but it’s only good for calculations involving water. If you need to deduce heating time for other fluids, this calculator is more suitable as it lets you enter the specific heat capacity of the substance you’re using. It has two options enabling you to calculate either power required or time required.
The Process Heating Services calculator.
Calculating Cooling Time
To calculate cooling time, you can use the same equation as above. The question is what value should you use for power. The cooling capacity (or cooling power) is different depending on the temperature. Cooling capacity decreases at lower setpoint temperatures because there’s a smaller temperature differential between the chiller liquid and refrigerant. Heat transfer is reduced so cooling capacity is lowered.
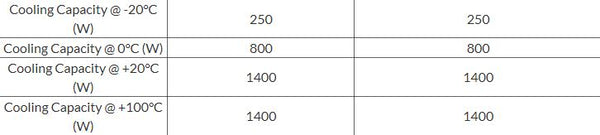
For example, here are the cooling capacity specifications for the PolyScience 45 L Refrigerated & Heated Circulating Baths.
You have a few options here depending on how to accurately you want your calculation to be:
- Use a conservative estimate by assuming the lower power up to the next listed temperature. For instance, taking the specifications above, you could assume that the cooling capacity is 250 W for all temperatures between -20°C and 0°C and 800 W for all temperatures between 0°C and 20°C.
- Potentially underestimate but with more accuracy by taking the average power between various temperatures.
- Use a quick and dirty (and likely less accurate) method by only considering the cooling capacity at the midpoint temperature.
- Opt for an alternative quick method that uses an average of cooling capacity values at various points in the temperature range (the points would need to include the upper and lower ends of the temperature range for this to be viable).
What if your minimum temperature is below the lowest temperature cooling capacity specification provided? This generally should not be a concern as cooling capacity values are typically provided for a temperature at or below the minimum temperature of the unit.
If you’re trying to cool to a lower temperature, it may be too low, meaning the unit won’t be able to provide the cooling capacity you need. However, if the specs don’t provide the cooling capacity at a temperature that is close to the minimum temperature of the unit, you can ask the manufacturer or us to provide the information you need.
Factors to Consider When Calculating Heating or Cooling Time
As mentioned, there are several reasons your calculations may not deliver a realistic result. As such, if you’re using this equation to determine heating or cooling time, you should assume that the process will take a little longer than expected. Similarly, if you’re using the calculation to determine how much power you need to achieve a given heating or cooling time, you should assume some additional power will be required.
Here are the factors you need to consider:
1. Ambient Heat Gain or Loss
Ambient heat loss gain or loss is inevitable, even in a closed system. A cooled system can absorb heat from the ambient air or system components, decreasing its cooling capacity. In a heating system, you may lose heat to the ambient air or to components of the system, for example, as it runs through tubes or pipes.
Insulating your system and controlling the ambient temperature can help, but there may still be an unknown amount of heat gain or loss.
2. Loss of Fluids to Evaporation
If you’re working with an open system, you may lose some fluids to evaporation during the heating or cooling process. The amount of evaporation that occurs will depend on several factors, including:
- Which fluid you’re using: Lower boiling point fluids such as ethanol, methanol, and water can evaporate easily.
- The surface area of the bath: The larger the surface area, the higher the rate of evaporation.
- The temperature range you are using: The higher the temperature, the higher the rate of evaporation.
Heat loss occurs through evaporation, and when you’re wasting heat energy, the time taken to heat the bath will increase. In addition, as a result of fluid loss, the mass value (m) in the equation won’t be accurate, potentially throwing off results. If you’re using a blend of two or more fluids and one component of a blend evaporates quicker than others, the ratio will be altered, leading to inaccuracy in the specific heat capacity (c).
Evaporation is difficult to predict and account for accurately (and if you are good enough with thermodynamics to be comfortable doing this, you probably wouldn’t be reading this article). As such, your best bets are to either estimate the evaporation rate through an empirical test and then factor that in mathematically using the heat of evaporation, or simply add a factor of safety.
3. Maintenance Issues
In heating systems, it’s common for scale to build up on the elements of a water bath due to mineral deposits. Left unchecked, this buildup can have an impact on the efficiency with which heat is transferred from the element to the fluid. With scale buildup insulating the element, more energy is required to heat the system to the desired temperature.
When heating, this will increase the time it will take to reach the desired temperature in a system of given power. If you’re looking at power, it will increase the amount of power required to reach the desired temperature in a certain amount of time.
For cooling systems, cooling capacity can be impacted by maintenance issues too. In water-cooled condensers, corrosion, scale buildup, or biological growth can inhibit heat transfer, lowering the cooling capacity. In air-cooled condensers, dust and debris buildup on fan blades and fins can decrease air flow, having a similar effect of lowering the cooling capacity.
Performing regular maintenance on your unit, including cleaning the various components, flushing the fluid, and using a corrosion inhibitor can help.